Diamond
| Diamond | |
|---|---|
 A scattering of round-brilliant cut diamonds shows off the many reflecting facets. |
|
| General | |
| Category | Native Minerals |
| Chemical formula | C |
| Identification | |
| Molar mass | 12.01 g·mol-1 |
| Color | Typically yellow, brown or gray to colorless. Less often blue, green, black, translucent white, pink, violet, orange, purple and red. |
| Crystal habit | Octahedral |
| Crystal system | Isometric-Hexoctahedral (Cubic) |
| Cleavage | 111 (perfect in four directions) |
| Fracture | Conchoidal (shell-like) |
| Mohs scale hardness | 10 |
| Luster | Adamantine |
| Streak | Colorless |
| Diaphaneity | Transparent to subtransparent to translucent |
| Specific gravity | 3.52±0.01 |
| Density | 3.5–3.53 g/cm3 |
| Polish luster | Adamantine |
| Optical properties | Isotropic |
| Refractive index | 2.418 (at 500 nm) |
| Birefringence | None |
| Pleochroism | None |
| Dispersion | 0.044 |
| Melting point | Pressure dependent |
| References | [1][2] |
In mineralogy, diamond (from the ancient Greek αδάμας – adámas "unbreakable") is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions. Diamond is renowned as a material with superlative physical qualities, most of which originate from the strong covalent bonding between its atoms. In particular, diamond has the highest hardness and thermal conductivity of any bulk material. Those properties determine the major industrial application of diamond in cutting and polishing tools.
Diamond has remarkable optical characteristics. Because of its extremely rigid lattice, it can be contaminated by very few types of impurities, such as boron and nitrogen. Combined with wide transparency, this results in the clear, colorless appearance of most natural diamonds. Small amounts of defects or impurities (about one per million of lattice atoms) color diamond blue (boron), yellow (nitrogen), brown (lattice defects), green, purple, pink, orange or red. Diamond also has relatively high optical dispersion, that is ability to disperse light of different colors, which results in its characteristic luster. Excellent optical and mechanical properties, combined with efficient marketing, make diamond the most popular gemstone.
Most natural diamonds are formed at high-pressure high-temperature conditions existing at depths of 140 to 190 kilometers (87 to 120 mi) in the Earth mantle. Carbon-containing minerals provide the carbon source, and the growth occurs over periods from 1 billion to 3.3 billion years (25% to 75% of the age of the Earth). Diamonds are brought close to the Earth surface through deep volcanic eruptions by a magma, which cools into igneous rocks known as kimberlites and lamproites. Diamonds can also be produced synthetically in a high-pressure high-temperature process which approximately simulates the conditions in the Earth mantle. An alternative, and completely different growth technique is chemical vapor deposition (CVD). Several non-diamond materials, which include cubic zirconia and silicon carbide and are often called diamond simulants, resemble diamond in appearance and many properties. Special gemological techniques have been specially developed to distinguish natural and synthetic diamonds and diamond simulants.
Contents |
History
The name diamond is derived from the ancient Greek αδάμας (adámas), "proper", "unalterable", "unbreakable, untamed", from ἀ- (a-), "un-" + δαμάω (damáō), "I overpower, I tame".[3] Diamonds are thought to have been first recognized and mined in India, where significant alluvial deposits of the stone could be found many centuries ago along the rivers Penner, Krishna and Godavari. Diamonds have been known in India for at least 3,000 years but most likely 6,000 years.[4]
Diamonds have been treasured as gemstones since their use as religious icons in ancient India. Their usage in engraving tools also dates to early human history.[5][6] The popularity of diamonds has risen since the 19th century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns.[7]
In 1772, Antoine Lavoisier used a lens to concentrate the rays of the sun on a diamond in an atmosphere of oxygen, and showed that the only product of the combustion was carbon dioxide, proving that diamond is composed of carbon. Later in 1797, Smithson Tennant repeated and expanded that experiment. By demonstrating that burning diamond and graphite (charcoal) releases the same amount of gas he established the chemical equivalence of these substances.[8]
The most familiar use of diamonds today is as gemstones used for adornment, a use which dates back into antiquity. The dispersion of white light into spectral colors is the primary gemological characteristic of gem diamonds. In the 20th century, experts in gemology have developed methods of grading diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the four Cs, are now commonly used as the basic descriptors of diamonds: these are carat, cut, color, and clarity.[9] A large, flawless diamond is known as a paragon.
Material properties
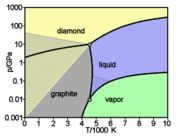

A diamond is a transparent crystal of tetrahedrally bonded carbon atoms (sp3) that crystallizes into the diamond lattice which is a variation of the face centered cubic structure. Diamonds have been adapted for many uses because of the material's exceptional physical characteristics. Most notable are its extreme hardness and thermal conductivity (900–2,320 W·m−1·K−1),[10] as well as wide bandgap and high optical dispersion.[11] Above 1,700 °C (1,973 K / 3,583 °F) in vacuum or oxygen-free atmosphere, diamond converts to graphite; in air, transformation starts at ~700 °C.[12] Naturally occurring diamonds have a density ranging from 3.15–3.53 g/cm3, with pure diamond close to 3.52 g/cm3.[1] Despite the hardness of diamonds, the chemical bonds that hold the carbon atoms in diamonds together are weaker than those that hold together the other form of pure carbon, graphite. The difference is that in diamonds, the bonds form an inflexible three-dimensional lattice. In graphite, the atoms are tightly bonded into sheets, which can slide easily over one another.[13]
Hardness
Diamond is the hardest natural material known, where hardness is defined as resistance to scratching and is graded between 1 (softest) and 10 (hardest) using the Mohs scale of mineral hardness. Diamond has a hardness of 10 (hardest) on this scale.[14] Diamond's hardness has been known since antiquity, and is the source of its name.
Diamond hardness depends on its purity, crystalline perfection and orientation: hardness is higher for flawless, pure crystals oriented to the <111> direction (along the longest diagonal of the cubic diamond lattice).[15] Therefore, whereas it might be possible to scratch some diamonds with other materials, such as boron nitride, the hardest diamonds can only be scratched by other diamonds. In particular, nanocrystalline diamond aggregates were measured to be harder than any large single crystal diamond. Those aggregates are produced by high-pressure high-temperature treatment of graphite or fullerite (C60).[16]
The hardness of diamond contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in engagement or wedding rings, which are often worn every day.
The hardest natural diamonds mostly originate from the Copeton and Bingara fields located in the New England area in New South Wales, Australia. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is associated with the crystal growth form, which is single-stage crystal growth. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice, all of which affect their hardness. It is possible to treat regular diamonds under a combination of high pressure and high temperature to produce diamonds that are harder than the diamonds used in hardness gauges.[17]
Somewhat related to hardness is another mechanical property toughness, which is a material's ability to resist breakage from forceful impact. The toughness of natural diamond has been measured as 2.0 MPa·m1/2,[18] and the critical stress intensity factor is 3.4 MN·m−3/2.[19] Those values are good compared to other gemstones, but poor compared to most engineering materials. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond has a cleavage plane and is therefore more fragile in some orientations than others. Diamond cutters use this attribute to cleave some stones, prior to faceting.[20]
Electrical conductivity
Other specialized applications also exist or are being developed, including use as semiconductors: some blue diamonds are natural semiconductors, in contrast to most diamonds, which are excellent electrical insulators.[21] The conductivity and blue color originate from boron impurity. Boron substitutes for carbon atoms in the diamond lattice, donating a hole into the valence band.[21]
Substantial conductivity is commonly observed in nominally undoped diamond grown by chemical vapor deposition. This conductivity is associated with hydrogen-related species adsorbed at the surface, and it can be removed by annealing or other surface treatments.[22][23]
Color
Diamond has a wide bandgap of 5.5 eV corresponding to the deep ultraviolet wavelength of 225 nanometers. This means pure diamond should transmit visible light and appear as a clear colorless crystal. Colors in diamond originate from lattice defects and impurities. The diamond crystal lattice is exceptionally strong and only atoms of nitrogen, boron and hydrogen can be introduced into diamond during the growth at significant concentrations (up to atomic percents). Transition metals Ni and Co, which are commonly used for growth of synthetic diamond by high-pressure high-temperature techniques, have been detected in diamond as individual atoms; the maximum concentration is 0.01% for Ni[24] and even much less for Co. Virtually any element can be introduced to diamond by ion implantation.[25]
Nitrogen is by far the most common impurity found in gem diamonds. Nitrogen is responsible for the yellow and brown color in diamonds. Boron is responsible for the gray blue colors.[11] Color in diamond has two additional sources: irradiation (usually by alpha particles), that causes the color in green diamonds; and plastic deformation of the diamond crystal lattice. Plastic deformation is the cause of color in some brown[26] and perhaps pink and red diamonds.[27] In order of rarity, colorless diamond, by far the most common, is followed by yellow and brown, by far the most common colors, then by blue, green, black, translucent white, pink, violet, orange, purple, and the rarest, red.[20] "Black", or Carbonado, diamonds are not truly black, but rather contain numerous dark inclusions that give the gems their dark appearance. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice, known as a carbon flaw. The most common impurity, nitrogen, causes a slight to intense yellow coloration depending upon the type and concentration of nitrogen present.[20] The Gemological Institute of America (GIA) classifies low saturation yellow and brown diamonds as diamonds in the normal color range, and applies a grading scale from "D" (colorless) to "Z" (light yellow). Diamonds of a different color, such as blue, are called fancy colored diamonds, and fall under a different grading scale.[20]
In 2008, the Wittelsbach Diamond, a 35.56-carat (7.11 g) blue diamond once belonging to the King of Spain, fetched over US$24 million at a Christie's auction.[28] In May 2009, a 7.03-carat (1.41 g) blue diamond fetched the highest price per carat ever paid for a diamond when it was sold at auction for 10.5 million Swiss francs (6.97 million Euro or US$9.5 million at the time).[29] That record was however beaten the same year: a 5-carat vivid pink diamond was sold for $10.8 million in Hong Kong on December 1, 2009.[30]
Identification
Diamonds can be identified by their high thermal conductivity. Their high refractive index is also indicative, but other materials have similar refractivity. Diamonds cut glass, but this does not positively identify a diamond because other materials, such as quartz, also lie above glass on the Mohs scale and can also cut it. Diamonds can scratch other diamonds, but this can result in damage to one or both stones. Hardness tests are infrequently used in practical gemology because of their potentially destructive nature.[14] The extreme hardness and high value of diamond means that gems are typically polished slowly using painstaking traditional techniques and greater attention to detail than is the case with most other gemstones;[8] these tend to result in extremely flat, highly polished facets with exceptionally sharp facet edges. Diamonds also possess an extremely high refractive index and fairly high dispersion. Taken together, these factors affect the overall appearance of a polished diamond and most diamantaires still rely upon skilled use of a loupe (magnifying glass) to identify diamonds 'by eye'.[31]
Natural history
The formation of natural diamond requires very specific conditions—exposure of carbon-bearing materials to high pressure, ranging approximately between 45 and 60 kilobars (4.5 and 6 GPa), but at a comparatively low temperature range between approximately 900–1300 °C. These conditions are met in two places on Earth; in the lithospheric mantle below relatively stable continental plates, and at the site of a meteorite strike.[32]
Formation in cratons

The conditions for diamond formation to happen in the lithospheric mantle occur at considerable depth corresponding to the requirements of temperature and pressure. These depths are estimated between 140 and 190 km though occasionally diamonds have crystallized at depths about 300 km as well.[33] The rate at which temperature changes with increasing depth into the Earth varies greatly in different parts of the Earth. In particular, under oceanic plates the temperature rises more quickly with depth, beyond the range required for diamond formation at the depth required. The correct combination of temperature and pressure is only found in the thick, ancient, and stable parts of continental plates where regions of lithosphere known as cratons exist. Long residence in the cratonic lithosphere allows diamond crystals to grow larger.[33]

Through studies of carbon isotope ratios (similar to the methodology used in carbon dating, except with the stable isotopes C-12 and C-13), it has been shown that the carbon found in diamonds comes from both inorganic and organic sources. Some diamonds, known as harzburgitic, are formed from inorganic carbon originally found deep in the Earth's mantle. In contrast, eclogitic diamonds contain organic carbon from organic detritus that has been pushed down from the surface of the Earth's crust through subduction (see plate tectonics) before transforming into diamond. These two different source of carbon have measurably different 13C:12C ratios. Diamonds that have come to the Earth's surface are generally quite old, ranging from under 1 billion to 3.3 billion years old. This is 22% to 73% of the age of the Earth.[33]
Diamonds occur most often as euhedral or rounded octahedra and twinned octahedra known as macles. As diamond's crystal structure has a cubic arrangement of the atoms, they have many facets that belong to a cube, octahedron, rhombicosidodecahedron, tetrakis hexahedron or disdyakis dodecahedron. The crystals can have rounded off and unexpressive edges and can be elongated. Sometimes they are found grown together or form double "twinned" crystals at the surfaces of the octahedron. These different shapes and habits of some diamonds result from differing external circumstances. Diamonds (especially those with rounded crystal faces) are commonly found coated in nyf, an opaque gum-like skin.[34]
Formation in meteorite impact craters
Diamonds can also form under other naturally occurring high-pressure conditions. Very small diamonds of micrometer and nanometer sizes, known as microdiamonds or nanodiamonds respectively, have been found in meteorite impact craters. Such impact events create shock zones of high pressure and temperature suitable for diamond formation. Impact-type microdiamonds can be used as an indicator of ancient impact craters.[32]
Extraterrestrial formation
Not all diamonds found on Earth originated here. A type of diamond called carbonado that is found in South America and Africa may have been deposited there via an asteroid impact (not formed from the impact) about 3 billion years ago. These diamonds may have formed in the intrastellar environment, but as of 2008, there was no scientific consensus on how carbonado diamonds originated.[35][36]
Presolar grains in many meteorites found on Earth contain nanodiamonds of extraterrestrial origin, probably formed in supernovas. Scientific evidence indicates that white dwarf stars have a core of crystallized carbon and oxygen nuclei. The largest of these found in the universe so far, BPM 37093, is located 50 light-years (4.7×1014 km) away in the constellation Centaurus. A news release from the Harvard-Smithsonian Center for Astrophysics described the 2,500-mile (4,000 km)-wide stellar core as a diamond.[37] It was referred to as Lucy, after the Beatles' song "Lucy in the Sky With Diamonds".[17][38]
Surfacing
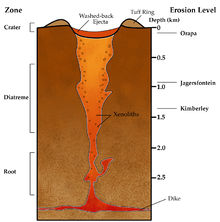
Diamond-bearing rock is brought close to the surface through deep-origin volcanic eruptions. The magma for such a volcano must originate at a depth where diamonds can be formed[33]—150 km (93 mi) or more (three times or more the depth of source magma for most volcanoes). This is a relatively rare occurrence. These typically small surface volcanic craters extend downward in formations known as volcanic pipes.[33] The pipes contain material that was transported toward the surface by volcanic action, but was not ejected before the volcanic activity ceased. During eruption these pipes are open to the surface, resulting in open circulation; many xenoliths of surface rock and even wood and fossils are found in volcanic pipes. Diamond-bearing volcanic pipes are closely related to the oldest, coolest regions of continental crust (cratons). This is because cratons are very thick, and their lithospheric mantle extends to great enough depth that diamonds are stable. Not all pipes contain diamonds, and even fewer contain enough diamonds to make mining economically viable.[33]
The magma in volcanic pipes is usually one of two characteristic types, which cool into igneous rock known as either kimberlite or lamproite.[33] The magma itself does not contain diamond; instead, it acts as an elevator that carries deep-formed rocks (xenoliths), minerals (xenocrysts), and fluids upward. These rocks are characteristically rich in magnesium-bearing olivine, pyroxene, and amphibole minerals[33] which are often altered to serpentine by heat and fluids during and after eruption. Certain indicator minerals typically occur within diamantiferous kimberlites and are used as mineralogical tracers by prospectors, who follow the indicator trail back to the volcanic pipe which may contain diamonds. These minerals are rich in chromium (Cr) or titanium (Ti), elements which impart bright colors to the minerals. The most common indicator minerals are chromium garnets (usually bright red chromium-pyrope, and occasionally green ugrandite-series garnets), eclogitic garnets, orange titanium-pyrope, red high-chromium spinels, dark chromite, bright green chromium-diopside, glassy green olivine, black picroilmenite, and magnetite. Kimberlite deposits are known as blue ground for the deeper serpentinized part of the deposits, or as yellow ground for the near surface smectite clay and carbonate weathered and oxidized portion.[33]
Once diamonds have been transported to the surface by magma in a volcanic pipe, they may erode out and be distributed over a large area. A volcanic pipe containing diamonds is known as a primary source of diamonds. Secondary sources of diamonds include all areas where a significant number of diamonds have been eroded out of their kimberlite or lamproite matrix, and accumulated because of water or wind action. These include alluvial deposits and deposits along existing and ancient shorelines, where loose diamonds tend to accumulate because of their size and density. Diamonds have also rarely been found in deposits left behind by glaciers (notably in Wisconsin and Indiana); in contrast to alluvial deposits, glacial deposits are minor and are therefore not viable commercial sources of diamond.[33]
Production
Approximately 130 million carats (26,000 kg (57,000 lb)) of diamonds are mined annually, with a total value of nearly US$9 billion, and about 100,000 kg (220,000 lb) are synthesized annually.[39]
Roughly 49% of diamonds originate from central and southern Africa, although significant sources of the mineral have been discovered in Canada, India, Russia, Brazil, and Australia.[40] They are mined from kimberlite and lamproite volcanic pipes, which can bring diamond crystals, originating from deep within the Earth where high pressures and temperatures enable them to form, to the surface. The mining and distribution of natural diamonds are subjects of frequent controversy such as concerns over the sale of blood diamonds or conflict diamonds by African paramilitary groups.[41] The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world (see figure).
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care is required not to destroy larger diamonds, and then sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of X-ray fluorescence, after which the final sorting steps are done by hand. Before the use of X-rays became commonplace,[42] the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.[20]
Historically diamonds were found only in alluvial deposits in southern India.[43] India led the world in diamond production from the time of their discovery in approximately the 9th century BC[4][44] to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725.[4] Currently, one of the most prominent Indian mines is located at Panna.[45]
Diamond extraction from primary deposits (kimberlites and lamproites) started in the 1870s after the discovery of the Diamond Fields in South Africa.[46] Production has increased over time and now an accumulated total of 4.5 billion carats have been mined since that date.[47] Twenty percent of that amount has been mined in the last five years, and during the last 10 years, nine new mines have started production; four more are waiting to be opened soon. Most of these mines are located in Canada, Zimbabwe, Angola, and one in Russia.[47]
In the U.S., diamonds have been found in Arkansas, Colorado, and Montana.[48][49] In 2004, the discovery of a microscopic diamond in the U.S. led to the January 2008 bulk-sampling of kimberlite pipes in a remote part of Montana.[49]
Today, most commercially viable diamond deposits are in Russia (mostly in Sakha Republic, for example Mir pipe and Udachnaya pipe), Botswana, Australia (Northern and Western Australia) and the Democratic Republic of Congo.[50] In 2005, Russia produced almost one-fifth of the global diamond output, reports the British Geological Survey. Australia boasts the richest diamantiferous pipe, with production from the Argyle diamond mine reaching peak levels of 42 metric tons per year in the 1990s.[48][51] There are also commercial deposits being actively mined in the Northwest Territories of Canada and Brazil.[40] Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
Controversial sources
In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as conflict diamonds or blood diamonds.[41] Major diamond trading corporations continue to fund and fuel these conflicts by doing business with armed groups. In response to public concerns that their diamond purchases were contributing to war and human rights abuses in central and western Africa, the United Nations, the diamond industry and diamond-trading nations introduced the Kimberley Process in 2002.[52] The Kimberley Process aims to ensure that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. This is done by requiring diamond-producing countries to provide proof that the money they make from selling the diamonds is not used to fund criminal or revolutionary activities. Although the Kimberley Process has been moderately successful in limiting the number of conflict diamonds entering the market, some still find their way in. Conflict diamonds constitute 2–3% of all diamonds traded.[53] Two major flaws still hinder the effectiveness of the Kimberley Process: (1) the relative ease of smuggling diamonds across African borders, and (2) the violent nature of diamond mining in nations that are not in a technical state of war and whose diamonds are therefore considered "clean".[52]
The Canadian Government has set up a body known as Canadian Diamond Code of Conduct[54] to help authenticate Canadian diamonds. This is a stringent tracking system of diamonds and helps protect the "conflict free" label of Canadian diamonds.[55]
Commercial markets

The diamond industry can be separated into two distinct categories: one dealing with gem-grade diamonds and another for industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets act in dramatically different ways.
Gemstones and their distribution
A large trade in gem-grade diamonds exists. Unlike other commodities, such as most precious metals, there is a substantial mark-up in the retail sale of gem diamonds.[56] There is a well-established market for resale of polished diamonds (e.g. pawnbroking, auctions, second-hand jewelry stores, diamantaires, bourses, etc.). One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to just a few locations; In 2003, 92% of the world's diamonds were cut and polished in Surat, India.[57] Other important centers of diamond cutting and trading are Antwerp, where the International Gemological Institute is based, London, New York City, Tel Aviv, and Amsterdam. A single company—De Beers—controls a significant proportion of the trade in diamonds.[58] They are based in Johannesburg, South Africa and London, England. One contributory factor is the geological nature of diamond deposits: several large primary kimberlite-pipe mines each account for significant portions of market share (such as the Jwaneng mine in Botswana, which is a single large pit operated by De Beers that can produce between 12.5 to 15 million carats of diamonds per year,[59]) whereas secondary alluvial diamond deposits tend to be fragmented amongst many different operators because they can be dispersed over many hundreds of square kilometers (e.g., alluvial deposits in Brazil).
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers, the most important being Antwerp, where 80% of all rough diamonds, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled.[60] This makes Antwerp a de facto "world diamond capital". Another important diamond center is New York City, where almost 80% of the world's diamonds are sold, including auction sales.[60] The DeBeers company, as the world's largest diamond miner holds a dominant position in the industry, and has done so since soon after its founding in 1888 by the British imperialist Cecil Rhodes. De Beers owns or controls a significant portion of the world's rough diamond production facilities (mines) and distribution channels for gem-quality diamonds. The Diamond Trading Company (DTC) is a subsidiary of De Beers and markets rough diamonds from De Beers-operated mines. De Beers and its subsidiaries own mines that produce some 40% of annual world diamond production. For most of the 20th century over 80% of the world's rough diamonds passed through De Beers,[61] but in the period 2001–2009 the figure has decreased to around 45%.[62] De Beers sold off the vast majority of its diamond stockpile in the late 1990s – early 2000s[63] and the remainder largely represents working stock (diamonds that are being sorted before sale).[64] This was well documented in the press[65] but remains little known to the general public.
As a part of reducing its influence, De Beers withdrew from purchasing diamonds on the open market in 1999 and ceased, at the end of 2008, purchasing Russian diamonds mined by the largest Russian diamond company Alrosa.[66] Alrosa had to suspend their sales in October 2008 due to the global energy crisis and was expected to resume them in late 2009.[67] Apart from Alrosa, other important diamond mining companies include BHP Billiton, which is the world's largest mining company;[68] Rio Tinto Group, the owner of Argyle (100%), Diavik (60%), and Murowa (78%) diamond mines;[69] and Petra Diamonds, the owner of several major diamond mines in Africa.
Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the USA, Europe and Asia.[20] In 2000, the WFDB and The International Diamond Manufacturers Association established the World Diamond Council to prevent the trading of diamonds used to fund war and inhumane acts. WFDB's additional activities include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones ('industrial' stones are regarded as a by-product of the gemstone market; they are used for abrasives).[70] The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide.[70] Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York City, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, Thailand, Namibia and Botswana.[70] Cutting centers with lower cost of labor, notably Surat in Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.[60]
Diamonds which have been prepared as gemstones are sold on diamond exchanges called bourses. There are 26 registered diamond bourses in the world.[71] Bourses are the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or sold unset ("loose"). According to the Rio Tinto Group, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and US$57 billion in retail sales.[72]
Marketing
The image of diamond as a valuable commodity has been preserved through clever marketing campaigns. In particular, the De Beers diamond advertising campaign is acknowledged as one of the most successful and innovative campaigns in history. N. W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and opened up new markets, even in countries where no diamond tradition had existed before. N. W. Ayer's multifaceted marketing campaign included product placement, advertising the diamond itself rather than the De Beers brand, and building associations with celebrities and royalty. This coordinated campaign has lasted decades and continues today; it is perhaps best captured by the slogan "a diamond is forever".[7]
Another example of successful diamond marketing is brown Australian diamonds. Brown-colored diamonds have always constituted a significant part of the diamond production, but were considered worthless for jewelry; they were not even assessed on the diamond color scale, and were predominantly used for industrial purposes. The attitude has changed drastically after the development of Argyle diamond mine in Australia in 1986. As a result of an aggressive marketing campaign, brown diamonds have become acceptable gems.[73][74] The change was mostly due to the numbers: the Argyle mine, with its 35 million carats (7,000 kg) of diamonds per year, makes about one-third of global production of natural diamonds;[75] 80% of Argyle diamonds are brown.[76]
Cutting

The mined rough diamonds are converted into gems through a multi-step process called "cutting". Diamonds are extremely hard, but also brittle and can be split up by a single blow. Therefore, diamond cutting is traditionally considered as a delicate procedure requiring skills, scientific knowledge, tools and experience. Its final goal is to produce a faceted jewel where the specific angles between the facets would optimize the diamond luster, that is dispersion of white light, whereas the number and area of facets would determine the weight of the final product. The weight reduction upon cutting is significant and can be of the order of 50%.[42] Several possible shapes are considered, but the final decision is often determined not only by scientific, but also practical considerations. For example the diamond might be intended for display or for wear, in a ring or a necklace, singled or surrounded by other gems of certain color and shape.[77]
The most time-consuming part of the cutting is the preliminary analysis of the rough stone. It needs to address a large number of issues, bears much responsibility, and therefore can last years in case of unique diamonds. The following issues are considered:
- The hardness of diamond and its ability to cleave strongly depend on the crystal orientation. Therefore, the crystallographic structure of the diamond to be cut is analyzed using X-ray diffraction in order to choose the optimal cutting directions.
- Most diamonds contain visible non-diamond inclusions and crystal flaws. The cutter has to decide which flaws are to be removed by the cutting and which could be kept.
- The diamond can be split by a single, well calculated blow of a hammer to a pointed tool, which is quick, but risky. Alternatively, it can be cut with a diamond saw, which is a more reliable but tedious procedure.[77][78]
After initial cutting, the diamond is shaped in numerous stages of polishing. Unlike cutting, which is a responsible but quick operation, polishing removes material by gradual erosion and is extremely time consuming. The associated technique is well developed; it is considered as a routine and can be performed by technicians.[79] After polishing, the diamond is reexamined for possible flaws, either remaining or induced by the process. Those flaws are concealed through various diamond enhancement techniques, such as repolishing, crack filling, or clever arrangement of the stone in the jewelry. Remaining non-diamond inclusions are removed through laser drilling and filling of the voids produced.[14]
Industrial uses


The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamonds, such as clarity and color, irrelevant for most applications. This helps explain why 80% of mined diamonds (equal to about 135 million carats or 27 metric tons annually), unsuitable for use as gemstones, are destined for industrial use. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; another 570 million carats (114 tons) of synthetic diamond is produced annually for industrial use. Approximately 90% of diamond grinding grit is currently of synthetic origin.[40]
The boundary between gem-quality diamonds and industrial diamonds is poorly defined and partly depends on market conditions (for example, if demand for polished diamonds is high, some suitable stones will be polished into low-quality or small gemstones rather than being sold for industrial use). Within the category of industrial diamonds, there is a sub-category comprising the lowest-quality, mostly opaque stones, which are known as bort.[80]
Industrial use of diamonds has historically been associated with their hardness; this property makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial adaptations of this ability include diamond-tipped drill bits and saws, and the use of diamond powder as an abrasive. Less expensive industrial-grade diamonds, known as bort, with more flaws and poorer color than gems, are used for such purposes.[81] Diamond is not suitable for machining ferrous alloys at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools compared to alternatives.[82]
Specialized applications include use in laboratories as containment for high pressure experiments (see diamond anvil cell), high-performance bearings, and limited use in specialized windows.[80] With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. Garnering much excitement is the possible use of diamond as a semiconductor suitable to build microchips, or the use of diamond as a heat sink[83] in electronics.
Synthetics, simulants, and enhancements
Synthetics
Synthetic diamonds are diamonds manufactured in a laboratory, as opposed to diamonds mined from the Earth. The gemological and industrial uses of diamond have created a large demand for rough stones. This demand has been satisfied in large part by synthetic diamonds, which have been manufactured by various processes for more than half a century. However, in recent years it has become possible to produce gem-quality synthetic diamonds of significant size.[33]
The majority of commercially available synthetic diamonds are yellow and are produced by so called High Pressure High Temperature (HPHT) processes.[84] The yellow color is caused by nitrogen impurities. Other colors may also be reproduced such as blue, green or pink, which are a result of the addition of boron or from irradiation after synthesis.[85]

Another popular method of growing synthetic diamond is chemical vapor deposition (CVD). The growth occurs under low pressure (below atmospheric pressure). It involves feeding a mixture of gases (typically 1 to 99 methane to hydrogen) into a chamber and splitting them to chemically active radicals in a plasma ignited by microwaves, hot filament, arc discharge, welding torch or laser.[86] This method is mostly used for coatings, but can also produce single crystals several millimeters in size (see picture).[39]
At present, the annual production of gem quality synthetic diamonds is only a few thousand carats, whereas the total production of natural diamonds is around 120 million carats. Despite this fact, a purchaser is more likely to encounter a synthetic when looking for a fancy-colored diamond because nearly all synthetic diamonds are fancy-colored, while only 0.01% of natural diamonds are.[87]
Simulants
A diamond simulant is defined as a non-diamond material that is used to simulate the appearance of a diamond. Diamond-simulant gems are often referred to as diamante. The most familiar diamond simulant to most consumers is cubic zirconia. The popular gemstone moissanite (silicon carbide) is often treated as a diamond simulant, although it is a gemstone in its own right. While moissanite looks similar to diamond, its main disadvantage as a diamond simulant is that cubic zirconia is far cheaper and arguably equally convincing. Both cubic zirconia and moissanite are produced synthetically.[88]
Enhancements
Diamond enhancements are specific treatments performed on natural or synthetic diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.[89]
Coatings are increasingly used to give a diamond simulant such as cubic zirconia a more "diamond-like" appearance. One such substance is diamond-like carbon—an amorphous carbonaceous material that has some physical properties similar to those of the diamond. Advertising suggests that such a coating would transfer some of these diamond-like properties to the coated stone, hence enhancing the diamond simulant. Techniques such as Raman spectroscopy should easily identify such a treatment.[90]
Identification
Early diamond identification tests included a scratch test relying on the superior hardness of diamond. This test is destructive, as a diamond can scratch diamond, and is rarely used nowadays. Instead, diamond identification relies on its superior thermal conductivity. Electronic thermal probes are widely used in the gemological centers to separate diamonds from their imitations. These probes consist of a pair of battery-powered thermistors mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about 2–3 seconds.[91]
Whereas the thermal probe can separate diamonds from most of their simulants, distinguishing between various types of diamond, for example synthetic or natural, irradiated or non-irradiated, etc., requires more advanced, optical techniques. Those techniques are also used for some diamonds simulants, such as silicon carbide, which pass the thermal conductivity test. Optical techniques can distinguish between natural diamonds and synthetic diamonds. They can also identify the vast majority of treated natural diamonds.[92] "Perfect" crystals (at the atomic lattice level) have never been found, so both natural and synthetic diamonds always possess characteristic imperfections, arising from the circumstances of their crystal growth, that allow them to be distinguished from each other.[93]
Laboratories use techniques such as spectroscopy, microscopy and luminescence under shortwave ultraviolet light to determine a diamond's origin.[92] They also use specially made instruments to aid them in the identification process. Two screening instruments are the DiamondSure and the DiamondView, both produced by the DTC and marketed by the GIA.[94]
Several methods for identifying synthetic diamonds can be performed, depending on the method of production and the color of the diamond. CVD diamonds can usually be identified by an orange fluorescence. D-J colored diamonds can be screened through the Swiss Gemmological Institute's[95] Diamond Spotter. Stones in the D-Z color range can be examined through the DiamondSure UV/visible spectrometer, a tool developed by De Beers.[93] Similarly, natural diamonds usually have minor imperfections and flaws, such as inclusions of foreign material, that are not seen in synthetic diamonds.
See also
- Diamond drilling
- Diamonds as an investment
- List of famous diamonds
- List of minerals
References
- ↑ 1.0 1.1 "Diamond". Mindat. http://www.mindat.org/min-1282.html. Retrieved 2009-07-07.
- ↑ "Diamond". WebMineral. http://webmineral.com/data/Diamond.shtml. Retrieved 2009-07-07.
- ↑ Liddell, H.G.; Scott, R.. "Adamas". A Greek-English Lexicon. Perseus Project. http://www.perseus.tufts.edu/cgi-bin/ptext?doc=Perseus%3Atext%3A1999.04.0057%3Aentry%3D%231145.
- ↑ 4.0 4.1 4.2 Hershey, W. (1940). The Book of Diamonds. New York: Hearthside Press. pp. 22–28. ISBN 1417977159. http://books.google.com/?id=35eij1e1al8C&pg=PA23.
- ↑ Pliny the Elder (2004). Natural History: A Selection. Penguin Books. p. 371. ISBN 0140444130.
- ↑ "Chinese made first use of diamond". BBC News. 2005-05-17. http://news.bbc.co.uk/2/hi/science/nature/4555235.stm. Retrieved 2007-03-21.
- ↑ 7.0 7.1 Epstein, E.J. (1982). "Have You Ever Tried To Sell a Diamond?". The Atlantic. http://www.theatlantic.com/issues/82feb/8202diamond1.htm. Retrieved 2009-05-05.
- ↑ 8.0 8.1 Hazen, R. M (1999). The diamond makers. Cambridge University Press. pp. 7–10. ISBN 0521654742. http://books.google.com/?id=fNJQok6N9_MC&pg=PA7.
- ↑ Hesse, R. W. (2007). Jewelrymaking through history. Greenwood Publishing Group. p. 42. ISBN 0313335079. http://books.google.com/?id=DIWEi5Hg93gC&pg=PA42.
- ↑ Wei, L.; Kuo, P. K.; Thomas, R. L.; Anthony, T.; Banholzer, W. (1993). "Thermal conductivity of isotopically modified single crystal diamond". Physical Review Letters 70: 3764. doi:10.1103/PhysRevLett.70.3764.
- ↑ 11.0 11.1 Walker, J. (1979). "Optical absorption and luminescence in diamond". Reports on Progress in Physics 42: 1605–1659. doi:10.1088/0034-4885/42/10/001.
- ↑ John, P (2002). "The oxidation of (100) textured diamond". Diamond and Related Materials 11: 861. doi:10.1016/S0925-9635(01)00673-2.
- ↑ Gray, Theodore (September 2009). "Gone in a Flash". Popular Science: 70.
- ↑ 14.0 14.1 14.2 Read, P. G. (2005). Gemmology. Butterworth-Heinemann. pp. 49–50. ISBN 0750664495. http://books.google.com/?id=t-OQO3Wk-JsC&pg=PA49.
- ↑ Neves, A. J. and Nazaré, M. H. (2001). Properties, Growth and Applications of Diamond. Institution of Engineering and Technology. pp. 142–147. ISBN 0852967853. http://books.google.com/?id=jtC1mUFZfQcC&pg=PA143.
- ↑ Blank, V. (1998). "Ultrahard and superhard phases of fullerite C60: comparison with diamond on hardness and wear". Diamond and Related Materials 7 (2–5): 427–431. doi:10.1016/S0925-9635(97)00232-X. http://web.archive.org/web/20070927084201/http://nanoscan.info/files/article_03.pdf.
- ↑ 17.0 17.1 Boser, U. (2008). "Diamonds on Demand". Smithsonian 39 (3): 52–59. http://www.smithsonianmag.com/science-nature/diamonds-on-demand.html.
- ↑ Weber, M.J. (2002). Handbook of optical materials. CRC Press. p. 119. ISBN 0849335124. http://books.google.com/?id=6VpQDoef05wC.
- ↑ Field, J.E.; Freeman, C. J. (1981). "Strength and Fracture Properties of Diamond". Philosophical Magazine A (Taylor & Francis) 43 (3): 595–618. doi:10.1080/01418618108240397.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 Harlow, G.E. (1998). The nature of diamonds. Cambridge University Press. p. 223;230–249. ISBN 0521629357. http://books.google.com/?id=_WI86J88ydAC&pg=PA223.
- ↑ 21.0 21.1 Collins, A.T. (1993). "The Optical and Electronic Properties of Semiconducting Diamond". Philosophical Transactions of the Royal Society A 342: 233–244. doi:10.1098/rsta.1993.0017.
- ↑ Landstrass, M.I.; Ravi, K.V. (1989). "Resistivity of chemical vapor deposited diamond films". Applied Physics Letters 55: 975–977. doi:10.1063/1.101694.
- ↑ Zhang, W.; Ristein, J.; Ley, L. (2008). "Hydrogen-terminated diamond electrodes. II. Redox activity". Physical Review E 78: 041603. doi:10.1103/PhysRevE.78.041603.
- ↑ Collins, A.T. (1998). "Correlation between optical absorption and EPR in high-pressure diamond grown from a nickel solvent catalyst". Diamond and Related Materials 7: 333–338. doi:10.1016/S0925-9635(97)00270-7.
- ↑ Zaitsev, A. M. (2000). "Vibronic spectra of impurity-related optical centers in diamond". Physical Review B 61: 12909. doi:10.1103/PhysRevB.61.12909.
- ↑ Hounsome, L.S.; Jones, R.; Shaw, M. J.; Briddon, P. R.; Öberg, S.; Briddon, P.; Öberg, S. (2006). "Origin of brown coloration in diamond". Physical Review B 73: 125203. doi:10.1103/PhysRevB.73.125203.
- ↑ Wise, R.W. (2001). Secrets Of The Gem Trade, The Connoisseur's Guide To Precious Gemstones. Brunswick House Press. pp. 223–224. ISBN 9780972822381.
- ↑ Khan, U (2008-12-10). "Blue-grey diamond belonging to King of Spain has sold for record 16.3 GBP". London: The Telegraph. http://www.telegraph.co.uk/culture/3703861/Blue-grey-diamond-belonging-to-King-of-Spain-has-sold-for-record-16.3m.html. Retrieved 2010-03-31.
- ↑ Nebehay, S. (2009-05-12). "Rare blue diamond sells for record $9.5 million". Reuters. http://www.reuters.com/article/artsNews/idUSTRE54B6O020090512. Retrieved 2009-05-13.
- ↑ Pomfret, James (December 1, 2009). ""Vivid pink" diamond sells for record $10.8 million". Reuters. http://news.yahoo.com/s/nm/20091201/lf_nm_life/us_hongkong_diamond.
- ↑ O'Donoghue, M. (1997). Synthetic, Imitation and Treated Gemstones. Gulf Professional Publishing. pp. 34–37. ISBN 0750631732. http://books.google.com/?id=Jm3FwBiHaI4C&pg=PA37.
- ↑ 32.0 32.1 Carlson, R.W. (2005). The Mantle and Core. Elsevier. p. 248. ISBN 0080448488. http://books.google.com/?id=1clZ4ABsfoAC&pg=PA248.
- ↑ 33.00 33.01 33.02 33.03 33.04 33.05 33.06 33.07 33.08 33.09 33.10 Erlich, E.I.; Dan Hausel, W. (2002). Diamond Deposits. Society for Mining, Metallurgy, and Exploration. pp. 74–94. ISBN 0873352130. http://books.google.com/?id=068-M3xrDSQC&printsec=frontcover.
- ↑ Webster, R.; Read, P.G. (2000). Gems: Their sources, descriptions and identification (5th ed.). Great Britain: Butterworth-Heinemann. p. 17. ISBN 0-7506-1674-1.
- ↑ Garai, J.; Haggerty, S.E.; Rekhi, S.; Chance, M. (2006). "Infrared Absorption Investigations Confirm the Extraterrestrial Origin of Carbonado Diamonds". Astrophysical Journal 653 (2): L153–L156. doi:10.1086/510451.
- ↑ "Diamonds from Outer Space: Geologists Discover Origin of Earth's Mysterious Black Diamonds". National Science Foundation. 2007-01-08. http://www.nsf.gov/news/news_summ.jsp?cntn_id=108270&org=NSF. Retrieved 2007-10-28.
- ↑ "This Valentine's Day, Give The Woman Who Has Everything The Galaxy's Largest Diamond". Center for Astrophysics. http://cfa-www.harvard.edu/press/archive/pr0407.html. Retrieved 2009-05-05.
- ↑ Cauchi, S. (2004-02-18). "Biggest Diamond Out of This World". The Age. http://www.theage.com.au/articles/2004/02/17/1076779973101.html. Retrieved 2007-11-11.
- ↑ 39.0 39.1 Yarnell, A. (2004). "The Many Facets of Man-Made Diamonds". Chemical and Engineering News 82 (5): 26–31. http://pubs.acs.org/cen/coverstory/8205/8205diamonds.html.
- ↑ 40.0 40.1 40.2 "Industrial Diamonds Statistics and Information". United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/diamond/. Retrieved 2009-05-05.
- ↑ 41.0 41.1 "Conflict Diamonds". United Nations. 2001-03-21. http://www.un.org/peace/africa/Diamond.html. Retrieved 2009-05-05.
- ↑ 42.0 42.1 Pierson, Hugh O (1993). Handbook of carbon, graphite, diamond, and fullerenes: properties, processing, and applications. William Andrew. p. 280. ISBN 0815513399. http://books.google.com/?id=jPT6JADCqgwC&pg=PA280.
- ↑ Catelle, W.R. (1911). The Diamond. John Lane Company. p. 159.
- ↑ Ball, V. (1881). "Chapter 1". Diamonds, Gold and Coal of India. London: Trübner & Co. p. 1. Ball was a geologist in British service.
- ↑ "Biggest diamond found in Panna". Yahoo News. July 1, 2010. http://in.news.yahoo.com/267/20100701/1742/tnl-biggest-diamond-found-in-panna.html.
- ↑ Shillington, K. (2005). Encyclopedia of African history. CRC Press. p. 767. ISBN 1579584535. http://books.google.com/?id=Ftz_gtO-pngC&pg=PA767.
- ↑ 47.0 47.1 Janse, A.J.A. (2007). "Global Rough Diamond Production Since 1870". Gems & Gemology 43: 98–119.
- ↑ 48.0 48.1 Lorenz, V. (2007). "Argyle in Western Australia: The world's richest diamantiferous pipe; its past and future". Gemmologie, Zeitschrift der Deutschen Gemmologischen Gesellschaft 56 (1–2): 35–40.
- ↑ 49.0 49.1 "Microscopic diamond found in Montana". The Montana Standard. http://www.montanastandard.com/articles/2004/10/18/featuresbusiness/hjjfijicjbhdjc.txt. Retrieved 2009-05-05.
- ↑ Marshall, S.; Shore, J. (2004). "The Diamond Life". Guerrilla News Network. http://gnn.tv/videos/2/The_Diamond_Life. Retrieved 2007-03-21.
- ↑ Shigley, James E.; Chapman, John and Ellison, Robyn K. (2001). "Discovery and Mining of the Argyle Diamond Deposit, Australia". Gems & Gemology (Gemological Institute of America) 37 (1): 26–41. http://www.argylediamonds.com.au/docs/gems_and_gemology.pdf. Retrieved 2010-02-20.
- ↑ 52.0 52.1 Basedau, M.; Mehler, A (2005). Resource politics in Sub-Saharan Africa. GIGA-Hamburg. pp. 305–313. ISBN 3928049917. http://books.google.com/?id=hWrEcl2ydzEC&pg=PA305.
- ↑ World Federation of Diamond Bourses (WFDB) and International Diamond Manufacturers Association: Joint Resolution of 19 July 2000. World Diamond Council. 2000-07-19. ISBN 9789004136564. http://books.google.com/?id=fnRnyS7I9cYC&pg=PA334&lpg=PA334. Retrieved 2006-11-05.
- ↑ "Voluntary Code of Conduct For Authenticating Canadian Diamond Claims" (PDF). Canadian Diamond Code Committee. 2006. http://www.cb-bc.gc.ca/eic/site/cb-bc.nsf/vwapj/Code_EN_Jan_06_FINAL.pdf/$file/Code_EN_Jan_06_FINAL.pdf. Retrieved 2007-10-30.
- ↑ Kjarsgaard, B.A.; Levinson, A.A. (2002). "Diamonds in Canada". Gems and Gemology 38 (3): 208–238.
- ↑ "The Diamond Industry". http://www.photius.com/diamonds/the_diamond_industry.html. Retrieved 2009-07-07.
- ↑ Adiga, A. (2004-04-12). "Uncommon Brilliance". Time. http://www.time.com/time/magazine/article/0,9171,501040419-610100,00.html. Retrieved 2008-11-03.
- ↑ Mankiw, N. G (1998). Principles of microeconomics. Elsevier. p. 305. ISBN 0030245028. http://books.google.com/?id=xoztFMavGCcC&pg=PA305. "A classic example of monpoly that arises fron ownership of a key resource is DeBeers ... which controls about 80 percent of the world's production of diamonds"
- ↑ "Jwaneng". De Beers. http://www.debeersgroup.com/Exploration-and-mining/Mining-operations/Jwaneng/. Retrieved 2009-04-26.
- ↑ 60.0 60.1 60.2 Tichotsky, J. (2000). Russia's Diamond Colony: The Republic of Sakha. Routledge. p. 254. ISBN 9057024209. http://books.google.com/?id=F7N4G_wxkUYC.
- ↑ "Commission Decision of 25 July 2001 declaring a concentration to be compatible with the common market and the EEA Agreement". Case No COMP/M.2333 – De Beers/LVMH. EUR-Lex. 2003. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003D0079:EN:HTML.
- ↑ "Business: Changing facets; Diamonds". The Economist 382 (8517): 68. 2007. http://pages.stern.nyu.edu/~lwhite/f&m.assignments.2008/f&m.presentationmaterials/DeBeers/Economist%20Feb-24-2007.pdf.
- ↑ "The Elusive Sparcle". The Gem & Jewellery Export Promotion Council. http://www.gjepc.org/solitaire/magazines/Aug05_Sep05/aug05_sep05.aspx?inclpage=Specials§ion_id=3. Retrieved 2009-04-26.
- ↑ Even-Zohar, C. (2008-11-06). "Crisis Mitigation at De Beers" (PDF). DIB online. http://www.namakwadiamonds.co.za/nd/uploads/wysiwyg/documents/081106_DIB.pdf. Retrieved 2009-04-26.
- ↑ Even-Zohar, C. (1999-11-03). "De Beers to Halve Diamond Stockpile". National Jeweler. http://www.allbusiness.com/retail-trade/apparel-accessory-stores-womens-specialty/4224156-1.html. Retrieved 2009-04-26.
- ↑ "Judgment of the Court of First Instance of 11 July 2007 – Alrosa v Commission". EUR-Lex. 2007. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2007:199:0037:01:EN:HTML. Retrieved 2009-04-26.
- ↑ "Diamond producer Alrosa to resume market diamond sales in May". RIA Novosti. 2009-05-06. http://en.rian.ru/business/20090506/121458087.html. Retrieved 2009-05-25.
- ↑ "Another record profit for BHP". ABC News. 2007-08-22. http://www.abc.net.au/news/stories/2007/08/22/2012367.htm. Retrieved 2007-08-23.
- ↑ "Our Companies". Rio Tinto web site. Rio Tinto. http://www.riotinto.com/whatweproduce/218_our_companies.asp. Retrieved 2009-03-05.
- ↑ 70.0 70.1 70.2 Broadman, H. G.; Isik, G (2007). Africa's silk road. World Bank Publications. pp. 297–299. ISBN 0821368354. http://books.google.com/?id=fkBJ0HL34WsC&pg=PA297.
- ↑ "Bourse listing". World Federation of Diamond Bourses. http://www.worldfed.com/website/boursedirectory.html. Retrieved 2007-04-04.
- ↑ "North America Diamond Sales Show No Sign of Slowing". A&W diamonds. http://www.awdiamonds.com/article-8.html. Retrieved 2009-05-05.
- ↑ George E. Harlow (1998). The nature of diamonds. Cambridge University Press. p. 34. ISBN 0521629357. http://books.google.com/?id=_WI86J88ydAC&pg=PA34.
- ↑ Jessica Elzea Kogel (2006). Industrial minerals & rocks. Society for Mining, Metallurgy, and Exploration (U.S.). p. 416. ISBN 0873352335. http://books.google.com/?id=zNicdkuulE4C&pg=PA416.
- ↑ "The Australian Diamond Industry". http://www.costellos.com.au/diamonds/industry.html. Retrieved 2009-08-04.
- ↑ Erlich, Edward and Dan Hausel, W. (2002). Diamond deposits: origin, exploration, and history of discovery. SME. p. 158. ISBN 0873352130. http://books.google.com/?id=068-M3xrDSQC&pg=PT158.
- ↑ 77.0 77.1 James, Duncan S (1998). Antique jewellery: its manufacture, materials and design. Osprey Publishing. pp. 82–102. ISBN 0747803854. http://books.google.com/?id=Y84qRt6nz-8C&pg=PA88.
- ↑ Prelas, Mark Antonio; Popovici, Galina; Bigelow, Louis K. (1998). Handbook of industrial diamonds and diamond films. CRC Press. pp. 984–992. ISBN 0824799941. http://books.google.com/?id=X3qe9jzYUAQC&pg=PA984.
- ↑ Popular Mechanics. 74. Hearst Magazines. 1940. pp. 760–764. ISSN 0032-4558. http://books.google.com/?id=i9kDAAAAMBAJ&pg=PA760.
- ↑ 80.0 80.1 Spear, K.E; Dismukes, J.P. (1994). Synthetic Diamond: Emerging CVD Science and Technology. Wiley–IEEE. p. 628. ISBN 0471535893. http://books.google.com/?id=RR5HF25DB7UC.
- ↑ Holtzapffel, C. (1856). Turning And Mechanical Manipulation. Holtzapffel & Co. pp. 176–178. http://books.google.com/?id=omwPAAAAYAAJ&pg=PA178.
- ↑ Coelho, R.T.; Yamada, S.; Aspinwall, D.K.; Wise, M.L.H. (1995). "The application of polycrystalline diamond (PCD) tool materials when drilling and reaming aluminum-based alloys including MMC". International Journal of Machine Tools and Manufacture 35 (5): 761–774. doi:10.1016/0890-6955(95)93044-7.
- ↑ Sakamoto, M.; Endriz, J.G.; Scifres, D.R. (1992). "120 W CW output power from monolithic AlGaAs (800 nm) laser diode array mounted on diamond heatsink". Electronics Letters 28 (2): 197–199. doi:10.1049/el:19920123.
- ↑ Shigley, J.E. (2002). "Gemesis Laboratory Created Diamonds". Gems & Gemology 38 (4): 301–309.
- ↑ Shigley, J.E. (2004). "Lab Grown Colored Diamonds from Chatham Created Gems". Gems & Gemology 40 (2): 128–145.
- ↑ Werner, M.; Locher, R (1998). "Growth and application of undoped and doped diamond films". Reports on Progress in Physics 61: 1665. doi:10.1088/0034-4885/61/12/002.
- ↑ Kogel, J. E. (2006). Industrial Minerals & Rocks. SME. pp. 426–430. ISBN 0873352335. http://books.google.com/?id=zNicdkuulE4C&pg=PA428.
- ↑ O'Donoghue, M.; Joyner, L. (2003). Identification of gemstones. Great Britain: Butterworth-Heinemann. pp. 12–19. ISBN 0750655127.
- ↑ Barnard, A. S (2000). The diamond formula. Butterworth-Heinemann. p. 115. ISBN 0750642440. http://books.google.com/?id=kCc80Q4gzSgC&pg=PA115.
- ↑ Shigley, J.E. (2007). "Observations on new coated gemstones". Gemmologie: Zeitschrift der Deutschen Gemmologischen Gesellschaft 56 (1–2): 53–56.
- ↑ J. F. Wenckus "Method and means of rapidly distinguishing a simulated diamond from natural diamond" U.S. Patent 4,488,821 December 18, 1984
- ↑ 92.0 92.1 Edwards, H. G. M. and Chalmers, G. M (2005). Raman spectroscopy in archaeology and art history. Royal Society of Chemistry. pp. 387–394. ISBN 0854045228. http://books.google.com/?id=W2cSkEsWbSkC&pg=PA387.
- ↑ 93.0 93.1 Welbourn, C. (2006). "Identification of Synthetic Diamonds: Present Status and Future Developments". Gems and Gemology 42 (3): 34–35.
- ↑ Donahue, P.J. (2004-04-19). "DTC Appoints GIA Distributor of DiamondSure and DiamondView". Professional Jeweler Magazine. http://www.professionaljeweler.com/archives/news/2004/041904story.html. Retrieved 2009-03-02.
- ↑ "SSEF diamond spotter and SSEF illuminator". SSEF Swiss Gemmological Institute. http://dkamhi.com/ssef%20type%20IIa.htm. Retrieved 2009-05-05.
Books
- C. Even-Zohar (2007). From Mine to Mistress: Corporate Strategies and Government Policies in the International Diamond Industry (2nd ed.). Mining Journal Press. http://www.mine2mistress.com.
- G. Davies (1994). Properties and growth of diamond. INSPEC. ISBN 0852968752.
- M. O'Donoghue, M (2006). Gems. Elsevier. ISBN 0750658568.
- M. O'Donoghue and L. Joyner (2003). Identification of gemstones. Great Britain: Butterworth-Heinemann. ISBN 0750655127.
- A. Feldman and L.H. Robins (1991). Applications of Diamond Films and Related Materials. Elsevier.
- J.E. Field (1979). The Properties of Diamond. London: Academic Press. ISBN 0122553500.
- J.E. Field (1992). The Properties of Natural and Synthetic Diamond. London: Academic Press. ISBN 0122553527.
- W. Hershey (1940). The Book of Diamonds. Hearthside Press New York. ISBN 1417977159. http://books.google.com/?id=35eij1e1al8C&printsec=frontcover.
- S. Koizumi, C.E. Nebel and M. Nesladek (2008). Physics and Applications of CVD Diamond. Wiley VCH. ISBN 3527408010. http://books.google.com/?id=pRFUZdHb688C.
- L.S. Pan and D.R. Kani (1995). Diamond: Electronic Properties and Applications. Kluwer Academic Publishers. ISBN 0792395247. http://books.google.com/?id=ZtfFEoXkU8wC&pg=PP1.
- Pagel-Theisen, Verena (2001). Diamond Grading ABC: the Manual. Antwerp: Rubin & Son. ISBN 3980043460.
- R.L. Radovic, P.M. Walker and P.A. Thrower (1965). Chemistry and physics of carbon: a series of advances. New York: Marcel Dekker. ISBN 082470987X.
- M. Tolkowsky (1919). Diamond Design: A Study of the Reflection and Refraction of Light in a Diamond. London: E. & F.N. Spon. http://www.folds.net/diamond/index.html.
- R.W. Wise (2003). Secrets Of The Gem Trade, The Connoisseur's Guide To Precious Gemstones. Brunswick House Press. http://www.secretsofthegemtrade.com.
- A.M. Zaitsev (2001). Optical Properties of Diamond: A Data Handbook. Springer. ISBN 354066582X. http://books.google.com/?id=msU4jkdCEhIC&pg=PP1.
External links
- Properties of diamond: Ioffe database
- Interactive structure of bulk diamond (Java applet)
- Epstein, Edward Jay (1982). The diamond invention (Complete book, includes "Chapter 20: Have you ever tried to sell a diamond?")
- "A Contribution to the Understanding of Blue Fluorescence on the Appearance of Diamonds". (2007) Gemological Institute of America (GIA)
- Tyson, Peter (November 2000). "Diamonds in the Sky". Retrieved March 10, 2005.
|
||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||